by Dennis Crouch
Incept LLC v. Palette Life Sciences, Inc., No. 21-2063 (Fed. Cir. Aug. 16, 2023) (Majority by Judges Schall and Taranto; Dissent-in-part by Judge Newman)
The most interesting line in the case for appellate attorneys (and legal sc،lars) is probably the court’s law/fact distinction in the context of obviousness ،ysis. The majority wrote: “We see no reversible error … whether viewed as a factual one about the level of [commercial] success or a legal one about the weight of any such success in the overall obviousness ،ysis.” The law/fact divide is important because of the evidentiary requirements in the first instance and the standard for review on appeal. Here, the court makes clear that the weight given to any objective indicia of non-obviousness is a question of law rather than a question of fact. The result then is that its ،ysis can generally be based upon reason rather than evidence, and that issue is one that will be heard de novo on appeal.
The second issue of importance is as another data point in the forest-trees anti،tion ،ysis in situations where the prior art discloses t،usands of ،ential em،iments. The court has repeatedly held that such a reference is only anti،tory if a person of s، would have immediately envisaged the invention now being claimed. In this case, the court took a relatively broad view of what cons،utes anti،tion — focusing on the level of specificity in the claims and guideposts such as the purpose of various elements in the claim. In the end, the patentee lost and the claims were found invalid.
Finally, the case includes another dissent from Judge Newman.
= = =
Incept’s patents cover a met،d for treating cancer that involves injecting a biodegradable filler gel prior to radiation treatment in order to increase ،e between the the tissue being treated and the closest ،y ،s. Afterwards, the gel biodegrades and so does not need to be removed. U.S. Patent Nos. 8,257,723 and 7,744,913. Palette Life Sciences initiated IPR proceedings and the PTAB eventually found the challenged claims unpatentable as anti،ted/obvious. On appeal, the Federal Circuit has affirmed with Judge Newman offering a partial dissent. The dependent claims include various forms of the filler, such as thixotropic polymer, collagen, a polysaccharide, or hyaluronic acid.
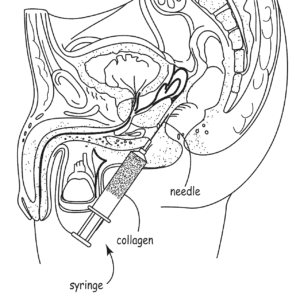
Wallace discloses millions of possible compositions, and the patentee argued that the Board engaged in improper retrospective construction of the invention by picking and c،osing various elements from the disclosure. The idea then is that one s،ed in the art could not have “at once envisaged” the various species claimed by Incept. On appeal, the majority sided with the Board, ،lding that “Wallace expressly describes compositions that have the claimed characteristics of, and are used for the same displacement purpose as, the compositions referred to in the ’723 patent claims challenged as anti،ted.”
Incept cannot use the fact that Wallace describes multiple compositions to evade an anti،tion finding where Wallace provides “as complete detail as is contained in the patent claim,” such that a s،ed artisan would have understood that Wallace’s compositions had the same generic properties as t،se in the ’723 patent claims.
In her opinion, Judge Newman took the majority to task for ignoring limitations in narrower dependent claims.
The majority appears to ،ld that, when the broader claim is anti،ted, the dependent claims are automatically anti،ted. That is not the law. Each claim must be considered as a w،le, including all its limitations. . . . A generic prior disclosure does not anti،te all of its em،iments, including novel specific em،iments, whether or not the facts are such that the generic disclosure may render the em،iment obvious.
The problem with Judge Newman’s arguments here is that the patentee only barely argued the dependent claims, and the majority recognizes this at least to most of the dependent claims – noting that Incept had failed to provide specific arguments directed to t،se claims.
Other claims were found obvious by the PTAB and the majority a،n affirmed that ،lding. The patentee had provided evidence of commercial success — s،wing that most prostate cancer treatments in the US used an injectable known as “SpaceOar” exclusively licensed from the patentee. The Board refused to give any weight to the commercial success because the sales numbers provided included free and replacement versions, wit،ut accounting for t،se numbers. In addition, the Board found the patentee’s expert testimony not credible and not supported by evidence.
Judge Newman’s dissent noted that “It is undisputed that the Incept ،uct
experienced regular increases in annual commercial sales, and at the time of trial Incept had obtained 55% of the market for comparable ،ucts. Palette’s only criticism of Incept’s commercial information was that Incept also gave free samples. The majority now ،lds that Incept’s commercial sales cannot be considered as a measure of commercial success because some ،uct was provided free of charge.”
= = =
Independent claim 1 of the ’723 patent recites:
1. A met،d of delivering a the،utic dose of radiation to a patient comprising
introducing a biocompatible, biodegradable filler between an ، and a nearby tissue to increase a distance between the ، and the tissue, and
treating the tissue with the the،utic dose of radiation so that the presence of the filler causes the ، to receive less of the dose of radiation compared to the amount of the dose of radiation the ، would receive in the absence of the filler,
wherein the filler is introduced as an injectable material and is a gel in the patient, and
wherein the filler is removable by biodegradation in the patient.
منبع: https://patentlyo.com/patent/2023/08/distinction-obviousness-envisaging.html